          


 |
|
Mathematical Modeling and Genetic Analysis of the Proneural Wave |
| |
In addition to molecular genetic studies, we also focus on mathematical biology researches. By combining mathematical modeling and genetic analysis, we can understantd the nature of complex phenomena that are very hard to understand using conventional approaches. By experimantally proving the predictions obtaind by the methematical models, we elucidate the novel functions of gene networks.
In the course of cell-cell communication, diffusible proteins act in a long range, while membrane proteins act in a short range. EGF and Notch play especially important roles for the long and short range cell-cell communications, respectively. However, how these two signaling systems behave when combined with each other remains elusive. We focused on the combined action of EGF and Notch during brain development. By experimentally proving the results of numerical simulations based on the mathematical model, we revealed that the roles of Notch significantly changes when combined with EGF function.
To investigate the co-operation of EGF and Notch, we focused on the wave of differentiation in the fly brain or 'proneural wave', which is similar to the wave of differentiation found in other organisms. The short range action of Notch usually forms the salt-and-pepper pattern. However, Notch does not form the salt-and-pepper pattern during the proneural wave propagation. According to our mathematical model, it was predicted that reduction in EGF production should cause the formation of the salt-and-pepper pattern. Surprisingly, partial reduction in EGF signaling caused the formation of salt-and-pepper pattern in vivo, suggesting that the short range action of Notch is indeed implemented in the proneural wave and that the combination of Notch and EGF enables a novel function of Notch signaling that regulates the propagation of the wave of differentiation.
The combinatorial actions of EGF and Notch are found in many biological processes including neural stem cell differentiation in the developing cerebral cortex and development of lund and breast cancers. Therefore, the mechanistic principles of gene network and the mathematical model established in this study might also be applied to the above biological processes in the future.
|
|
| Notch-mediated lateral inhibition and reaction diffusion of EGF |
|
|
In this study, we focused on two signaling systems, EGF and Notch. EGF is a diffusible factor that act in a long range, while Notch is activated by a membrane bound ligand that act in a short lange. The short range action of Notch is called lateral inhibition. In the above figure a., blue cells are differentiated to red cells. As red cells send an inhibitory signal to the neighboring cells, blue cells are always situated adjacent to red cells, which is so called salt-and-pepper pattern. On the other hand, EGF has a long range effect that acts on cells distant from the EGF producing cells (figure b).
Although both of them play very important roles in many biological processes, the combinatorial action of these two signaling systems remains elusive.
To investigate the co-operative functions of EGF and Notch, we focused on the wave of differentiation in the fly brain or 'proneural wave'. In the developing fly brain, all of the cells are undifferentiated neuroepithelial cells (blue cells), which are sequentially differentiated to neuroblast, a kind of neural stem cell (red cell), following the progress of the proneural wave (figure c). It has been shown that the proneural wave propagation is positively and negatively controlled by EGF and Notch, respectively.
The Notch-mediated lateral inhibition is found in many biological processes of wide variety of animals. Furthermore, the conditions required for Notch-mediated lateral inhibition are fulfiled in the proneural wave. If Notch-mediated lateral inhibition is included in the proneural wave, the red and blue cells should show the salt-and-pepper pattern shown in figure a. However, no such pattern has been found in the proneural wave (figure c). So, the questions are as follow: Is Notch-mediated lateral inhibition included in the proneural wave? If so, why Notch does not couse the salt-and-pepper pattern, but controls the proneural wave propagation?
Conventional biological approaches are not sufficient to answer these questions. We therefore established a mathematical model of the proneural wave. By combining the computer simulation and molecular genetic experiments, we investigated the dynamics of the gene network including EGF and Notch (figure d).
|
|
|
| Salt-and-Pepper pattern is revealed by reducing EGF production rate |
|
| |
Firstly, we assumed that the proneural wave include the Notch-mediated lateral inhibition and formulated a mathematical model by combining the reaction diffusion of EGF and Notch-mediated lateral inhibition. Interestingly, the model can very nicely recapitulate many characteristic behaviors of the proneural wave (figure a, c). In a wild type condition, the proneural wave propagates without showing the salt-and-pepper pattern. The differentiation is abolished in EGF mutant condition, while the wave progression is accelerated in Notch mutant condition. Thus, the simple combination of reaction diffusion of EGF and Notch-mediated lateral inhibition can clearly reproduce the complex behaviors of the proneural wave.
Then, what happened to the salt-and-pepper pattern? It is possible to assume that the stochastic activation pattern of Notch was canceled by the diffusible effect of EGF. According to the computer simulation based on the matimatical model, the salt-and-pepper pattern is revealed by reducing the EGF production rate (figure b). We tested this idea in vivo using RNAi to partially reduce EGF signaling, because the complete loss of EGF signaling causes the absence of the proneural wave. As a result, we demonstrated that the salt-and-pepper patterns in Notch activity and neuroblast differentiation were revealed when EGF signaling is reduced as predicted from the mathematical model (figure d). These results suggest that our model is appropriate and that the Notch-mediated lateral inhibition is indeed included in the proneural wave in vivo.
|
|
|
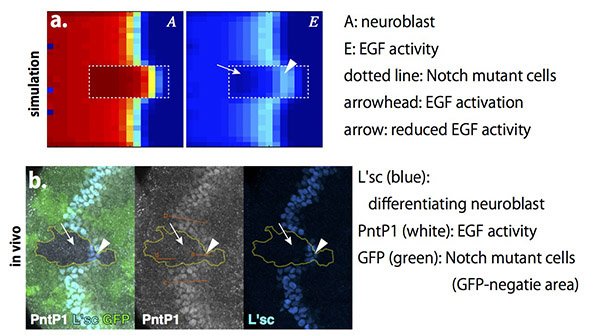
Temporal EGF activation causes wave acceleration in Notch mutant area
 |
| |
We tried to solve one more unsolved problem by using our model. It was reported that EGF activity is lost in Notch mutant regions, in which wave progression is accelerated. Since EGF is essential for the wave progression, the wave should be lost when EGF activity is lost. However, the wave is not lost, but instead accelerated, in Notch mutant cells, in which EGF is inactivated. The previous model of the proneural wave could not explain this paradox.
We generated Notch mutant area in the above mathematical model (figure a). Interestingly,when the wave is accerelated in the Notch mutant area, EGF activity was temporally upregulated, but was immediately inactivated. Since Notch represses neuroblast differentiation, loss of Notch in the presence of EGF activation should cause acceleration in neuroblast differentiation. To test this possibility in vivo, we induced a group of cells in which Notch signaling is abolished, and found that EGF activity is temporally up-regulated as deomnstrated by the computer simulation (figure b). Thus, we could clarify an unsolved paradox by combining the mathematical model and genetic analysis.
|
|
|
Jak/Stat guarantees robust neural stem cell differentiation by shutting off biological noise
| |
In addition to EGF and Notch, Jak/Stat signaling plays an essential role by repressing the progression of the proneural wave (Yasugi et al., Development, 135: 1471-80, 2008.). Jak/Stat is activated in undifferentiated neuroepithelial cells, and its absence causes acceleration of the wave propagation. By combining mathematical modeling and molecular genetic experiments, we further revealed that Jak/Stat has an additional function of noise cancelling.
|
|
The previous model became noise-resistant by incorporating the function of Jak/Stat
| |
Our previous mathematical model was not resistant to noise, because addition of mild noise to neuroepithelial cells causes expansion of abnormal neuroblast differentiation (top). However, simple addition of Jak/Stat function to the mathematical model canceled the noise effect and preroduced the normal pattern of proneural wave progression (bottom).
|
|
Reduction of Jak/Stat activity in the brain caused stochastic neuroblast differentiation
| |
In normal brain, the proneural wave progresses uniformly showing a smooth boundary (light blue in left). However, when Jak/Stat activity was uniformly repressed in the neuroepithelial cells, neuroblasts were stochastically differentiated and the regular pattern of proneural wave was disrupted. Thus, we revealed that Jak/Stat has a noise canselling function during brain development by combining mathematical modeling and molecular genetic experiments.
|
|
The interdiciplinary researches that combine biology and mathematics are essential for the further progress of the life sciences. Nevertheless, it is still very difficult to perform a true interdiciplinary research by mutulally combining biological experiments and numerical simulations. We established a mathematical model based on the experimental results, and confirmed its validity by genetic experiments. Additionally, we also experimentally proved the prediction of the model, and revealed the mechanism of the gene network including EGF, Notch and Jak/Stat.
The combinatorial actions of EGF and Notch are found in many biological processes including neural stem cell differentiation in the developing cerebral cortex and development of lung and breast cancers. Jak/Stat is also an evolutionarily conserved signaling pathway, and plays important roles in various stem cell systems in fly, mouse and human. Thus, Jak/Stat may also cancel biological noises in neural stem cells in mammalian cerebral cortex, ES cells and various stem cell systems. Therefore, the mechanistic principles of gene network and the mathematical model established in this study might also be applied to the wide variety of biological processes in the future.
Sato, M., Yasugi, T., Minami, Y., Miura, T. and Nagayama, M.
Notch-mediated lateral inhibition regulates proneural wave propagation when combined with EGF-mediated reaction diffusion. Proceedings of the National Academy of Sciences 113, E5153-E5162 (2016).
Tanaka, Y., Yasugi, T.,Nagayama, M., Sato, M. and Shin-Ichiro Ei.
JAK/STAT guarantees robust neural stem cell di erentiation by shutting off biological noise.
Scientific Reports 8, 12484 (2018).
|